Under steady-state conditions, the initial rate
of the reaction (vo) is given by the Michaelis-
Menten equation:
Compounds called inhibitors can interfere with the reaction. Inhibition can be competitive, uncompetitive, or of mixed type.
Competitive Inhibition
A competitive inhibitor, I, prevents the binding of substrate to the free enzyme, E, presumably by binding to the enzyme's active site. Thus, a competitive inhibitor competes directly with substrate for an enzyme's active site.
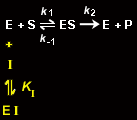
The inhibitor binds to the enzyme with the equilibrium constant KI:
In competitve inhibition, the Michaelis-Menten equation takes the form:
where a = 1 + [I]/KI. Hence, KM(app) = aKM, where KM(app) is the apparent value of KM. The Lineweaver-Burk equation then takes the form:
This describes a family of lines of slope aKM/Vmax that all intersect the 1/vo axis at 1/Vmax and have extrapolated intercepts on the 1/[S] axis of -1/aKM.
Uncompetitive Inhibition
An uncompetitive inhibitor, I, binds to the enzyme-substrate complex ES but not to the free enzyme, E.
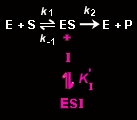
Its binding prevents catalysis (ESI is catalytically inactive), presumably by altering the enzyme's active site. An uncompetitive inhibitor binds to the enzyme with the equilibrium constant K'I:
In uncompetitive inhibition, the Michaelis-Menten equation takes the form:
where a' = 1 + [I]/K'I. Hence, KM(app) = KM/a' and Vmax(app) = Vmax/a', where KM(app) and Vmax(app) are the apparent values of KM and Vmax.
The Lineweaver-Burk equation then takes the form:
This describes a family of parallel lines of slope KM/Vmax, whose intercepts on the 1/vo axis are a'/Vmax and whose extrapolated intercepts on the 1/[S] axis are -a'/ KM.
Mixed Inhibition
A mixed inhibitor (alternatively, noncompetitive inhibitor), I, binds to both the enzyme, E, and to the enzyme-substrate complex, ES, thereby preventing the catalytic reaction.
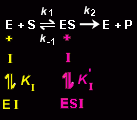
The inhibitor binds to the free enzyme with the equilibrium constant, KI:
and to the enzyme-substrate complex with the equilibrium constant K'I:
where KI and K'I are not necessarily equal.
In mixed inhibition, the Michaelis-Menten equation takes the form:
where a = 1 + [I]/KI and a' = 1 + [I]/K'I. Hence
KM(app) = aKM/a' and Vmax(app) = Vmax/a', where KM(app) and Vmax(app) are the apparent values of KM and Vmax.
The Lineweaver-Burk equation then takes the form:
This describes a family of lines that intersect to the left of the 1/vo axis (on the 1/[S] axis in the special case that a = a'), whose intercepts on the 1/vo axis are a'/Vmax and whose extrapolated intercepts on the 1/[S] axis are -a'KM/a.