|
BUFFERSIn the previous section, the Henderson–Hasselbalch equation was introduced, which is handy for dealing with pH calculations involving weak acids and their conjugate bases. This equation is very important to biochemists, who are always concerned with keeping proteins and other biological molecules in the lab at their proper pH. Indeed, a solution that contains a weak acid and its conjugate base has the special property of being able to resist changing its pH when either a base or an acid is added to it. Such a solution is called a buffer because the solution is protected, or buffered, from pH changes even when H3O+ or OH– ions are added to the solution. Buffers help biochemists study biomolecular reactions in the laboratory by stabilizing the pH of solutions used for experiments. To get a better sense of the utility of buffer solutions, let’s compare the difference between adding a strong base to a buffered solution, and adding the same amount of strong base to water. This is done in the examples below. Example 5
If 10 mL of 1M NaOH are added to one liter of a buffer that is 0.3 M acetic acid and 0.2 M sodium acetate (Na+CH3COO–), how much does the pH change? The pKa for acetic acid is 4.76. Answer:In the original solution, acetic acid is the weak acid, and acetate is the conjugate base. Thus, [base] = 0.2 M, and [acid] = 0.3 M. Using the Henderson–Hasselbalch equation,
NaOH is a strong base, and dissociates completely. Therefore, adding the strong base results in (0.01 liter)(1 M) = 0.01 moles of OH– ions into the solution. All of the OH– ions will react with the acetic acid to form acetate ions: CH3COOH + OH– Thus, when NaOH is added, the number of acetic acid molecules in the solution will decrease, but the number of acetate molecules in the solution will increase. In this case, 0.01 moles of acetic acid will be used up to neutralize the 0.01 moles of OH–, and this will form 0.01 moles of acetate ion in the solution. At the end of the reaction:
Now we are almost ready to plug back into the Henderson–Hasselbalch equation to find the new pH of the solution. We just need to remember that the volume has changed slightly, from 1 liter (1000 mL) to 1 liter plus 10 mL (1010 mL). The Henderson–Hasselbalch equation requires that the [Base] and [Acid] be molar (M, or mol/L) quantities. However, notice how that because both the acid and base species are in the same volume, the volume correction cancels out:
Example 6
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
(0.0099)[H3O+] = 10–14 |
Then plugging into the pH equation:
|
pH = – log [H+] |
The pH has shifted from the neutral pH of 7 for the pure water to a pH of almost 12 with the NaOH added, a shift of 5 pH units!
Buffering agents stabilize the pH of aqueous solutions
In example 5, the pH of the buffer solution changed by less than 0.5 units when the NaOH was added. Yet adding the same amount of NaOH to pure water resulted in a 5 unit pH shift in example 6. Clearly, the conjugate acid/base pair was able to protect the solution from a large change in pH.
A buffer is a solution that resists a change in pH when acids or bases are added to it.
How is this possible? Buffers work by acting a little bit like a sponge, soaking up excess H3O+ or OH– ions when they are added to a solution. The dissociation reaction for acetic acid shows that a solution of acetic acid contains acetate ions:
| CH3COOH + H2O |
How do acetic acid and the acetate ion work to buffer the solution? If a strong acid, such as hydrochloric acid, is added to this buffer, the H3O+ ions generated will react with the acetate ions, removing them from the solution:
| CH3COO– | + | H3O+ | CH3COOH | + | H2O | |
| acetate ion |
hydronium ion |
acetic acid |
water |
Similarly, if a strong base, generating lots of OH– ions, were added to the acetic acid buffer solution
| CH3COOH | + | OH– | CH3COO– | + | H2O | |
| acetic acid |
hydroxide ion |
acetate acid |
water |
The OH– ions are removed via a reaction with acetic acid. It is important to remember that buffers cannot maintain their pH indefinitely as more acid or base is added. Imagine slowly pouring a bucketful of water onto a small sponge on the kitchen floor. At first, as the water pours out of the bucket it is absorbed by the sponge, keeping the kitchen floor dry. But once the sponge is soaked through, it can hold no more and the water spills onto the floor as fast as if the sponge weren’t there. In the same way, buffers will protect the pH of the solution to some extent, but if they are inundated by large amounts of H3O+ or OH– ions, all the available conjugate acid or base molecules will have been used up and pH changes will rapidly occur.
Thus, buffers only work as long as the amount of conjugate acid and base ions are large compared to the H3O+amount of or OH– ions to be removed. Remember from the Henderson-Hasselbalch equation (section 5) that the numbers of conjugate acid molecules is equal to the number of conjugate base molecules when the pH = pKa:
| pH = pKa + log ( [Base] / [Acid] ) if [Base] = [Acid], then pH = pKa + log 1 pH = pKa |
Because the conjugate acid and conjugate base molecules are available in equal amounts when the pH = pKa, the buffer solution has the strongest ability to protect against pH changes caused by incoming H3O+ or OH– ions when the pH of the solution is close to the pKa of the conjugate acid. If the pH of the solution strays too far from the pKa point, the buffer uses up all its available conjugate acid or base molecules, and the pH starts to fall (or rise) dramatically. At this point, the buffering capacity of the buffer has been exceeded. this can be seen in the buffer (weak acid) titration graph below.
Buffers protect the pH of a solution best within one pH unit of the pKa.
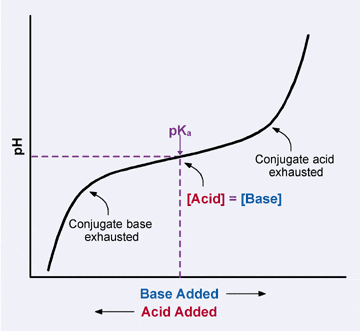 |
| Buffer titration: within one unit of the pKa, the solution resists changes in pH. |
Buffers are important for biochemistry because the structures (and therefore the functions) of biological molecules are stable within a relatively narrow range of pH values.The evolution of life, which is believed to have begun in water, was likely due in part to the stable pH of seawater. Even today, the largest buffered systems in the world are the Earth's oceans. Carbon dioxide (CO2) from the Earth’s atmosphere reacts with H2O to produce carbonic acid, H2CO3. Carbonic acid in turn reacts with water to form bicarbonate:
|
H2CO3 |
+ |
H2O |
HCO3– |
+ |
H3O+ |
|
|
carbonic |
water |
bicarbonate |
hydronium |
Furthermore, the bicarbonate ion can also react with water to form carbonate:
|
HCO3– |
+ |
H2O |
CO32– |
+ |
H3O+ |
|
|
bicarbonate |
water |
carbonate |
hydronium |
The carbonate and bicarbonate ions act together as conjugate acid/base pair to keep the pH of the ocean at about 8.2.
In the living organisms, proteins (the molecules which do most of the “work” of a cell) are very sensitive to acid concentration, because they only fold into their proper three-dimensional shapes within a small pH range. Some of the individual amino acid building blocks that make up a protein have ionizable side chains that change their ionization state, or charge, at different pH. Thus, if the pH changes, some of the attractive forces that hold the protein together will also change.
The ionization states of the side chain of the amino acid lysine are shown below. The pKa of lysine's side chain amino group is approximately 10.8. Therefore, at a pH lower than the pKa, the side chain is in the protonated (conjugate acid) state; whereas at a pH higher than the pKa, the side chain is unprotonated (conjugate base).
 |
| Amino acid charge changes: if the pH of the environment around a basic amino acid shifts so that it is greater than the pKa, the amino acid is deprotonated, no longer carries a charge, and is unable to form ionic bonds. |
Proteins consist of long chains of amino acids, many of which include acidic or basic groups. These groups are chaged at physiological pH, and form ionic interactions with each other that contribute to the stability of the folded protein. A change in pH of the protein's aqueous environment may alter the charge of some of these charged groups, disrupting the ionic interactions and the stability of the protein’s structure.
Remember that the Henderson–Hasselbalch equation was used for calculating the pH of a solution containing a weak acid and its conjugate base. Since such a solution is a buffer, the Henderson–Hasselbalch equation is extremely useful for calculations involving buffers, allowing the following:
- Determination of the pH of buffered solutions.
- Determination of the ratio of conjugate base to conjugate acid at a given pH.
Example 7

A solution of lactic acid has twice as many conjugate acid molecules as conjugate base molecules. If the pKa of lactic acid is 3.85, what is the pH of such a solution?
Answer:
We are given that the concentration of conjugate acid is twice the concentration of conjugate base. Thus,
[acid] = 2 [base]
Substituting into the Henderson-Hasselbalch equation:
|
pH = pKa + log
([base]/[acid]) |