| |
GIBBS FREE ENERGY
A thermodynamic indicator of success
We need to appreciate the principles of thermodynamics to understand how biochemical
reactions occur. Section 1 explained how the first two laws of thermodynamics
dictate the flow of energy through our universe. But how can we understand biochemical
reactions in terms of these laws of energy flow? In the 1800s, a scientist named
J. Willard Gibbs described a new value, “free energy,” to helps
us to do exactly that. Gibbs Free Energy, denoted by G, describes the
energy available to do work within a system, in this case a chemical reaction.
Free Energy can be used to make things occur that wouldn’t happen without
a source of energy. Examples of work being done include the progress of a chemical
reaction to create a new product, an engine running to turn the wheels of a
car, and falling water moving the turbines of a hydroelectric power plant. Keep
in mind that Free Energy, which is useful because it can be harnassed to do
work, is different from the heat energy that is always lost in any process.
This heat energy is generated as the cost of doing work, and is therefore often
called nature’s “heat tax.” The Gibbs Free Energy Change,
or DG, is the difference between the G
of the reactants and the G of the products.
Thus, the Gibbs Free Energy of reaction is the maximum potential usable
energy of a system, and is calculated by the following formula:
| DG = (energy of products)
– (energy of reactants) |
|
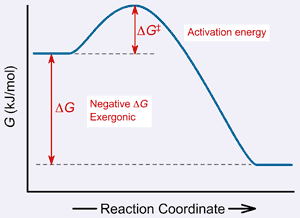 |
| Energy diagram: The free energy profile of a reaction going
from products to reactants. |
Here is a reaction diagram that illustrates DG.
If the DG is negative, meaning the products
are at a lower energy than the reactants, then the reaction is thermodynamically
favorable, meaning it can proceed. Remember, the second law of thermodynamics
states that a system will always want to move toward a lower energy state. Thus,
DG is useful because it tells us if a chemical
reaction is likely to proceed in the direction it is written.
However, the DG does not tell us at all
how fast this reaction will happen. This is because there is another quantity
that is important in the energetics of a chemical reaction. Take another look
at the energy diagram (above). Notice that as the reaction proceeds, the reactants
actually have to move through a higher energy state before they can arrive at
the low energy state of the products. In other words, the reactants have to scale
an “energy wall,” called the Energy of Activation ( DG‡).
The higher the DG‡, the taller
the wall. As you might suspect, a taller wall is more difficult to scale, and
thus reactions with a high DG‡
proceed slowly. In contrast, reactions with a low DG‡
progress more quickly. Such reactions are said to be kinetically favorable,
or likely to proceed rapidly. (For more on biochemical kinetics, see the kinetics
review.)
Example 3: Thermodynamic vs. kinetic stability

Blood collected 20 years ago from a crime scene is being used for DNA testing
at a murder trial to prove the suspect was indeed at the scene of the crime.
The defense lawyer argues that this test cannot be reliable, since she knows
that the hydrolysis reaction (breakdown) of DNA has a negative DG.
She concludes that therefore the DNA degraded spontaneously after all this
time, and is now useless. As an expert witness for the prosecution, how can
you use your knowledge of biochemistry to explain why she is mistaken?
Answer
You point out that a negative DG just
means the breakdown of DNA is thermodynamically favorable. You clarify this
by explaining that the DG value does not
say anything about how fast the reaction will occur. In fact, DNA is kinetically
stable, and thus the breakdown of DNA happens very slowly, making DNA evidence
valid decades after crimes have been commited.
The figure above shows a reaction with a negative DG
(the products have less free energy than the reactants). Such an energy-releasing
reaction is said to be exergonic. However, some reactions have a positive DG
(where products have more free energy than the starting reactants). As you might
imagine, such reactions require energy input, and are called endergonic, or
energy consuming.
| GIBBS
FREE ENERGY |
| Sign of DG |
Energy Transferred |
Term Used |
| Positive DG |
Consumes free energy
(Requires energy input to proceed) |
Endergonic |
| Negative DG |
Releases free energy
(No energy input required to proceed) |
Exergonic |
| DG = 0 |
No free energy transferred
(Does not proceed) |
At equilibrium
|
|
Keep in mind that energy released in an exergonic reaction is not necessarily
released as heat. Thus, an exergonic (energy releasing) reaction is not necessarily
exothermic (heat releasing). In other words, a reaction with a negative DG
(free energy) value may have either a positive or negative DH
(enthalpy) value!
|

