|
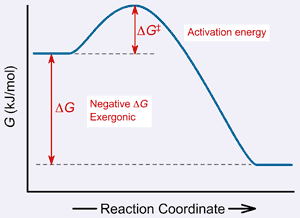
REACTION SPONTANEITYMix some baking soda (sodium bicarbonate) and vinegar (acetic acid) in a glass in the kitchen and you’ll see an energetic reaction that releases carbon dioxide gas bubbles and makes a frothy mess on the counter. Mix some flour (carbohydrates) with the vinegar and all you get is…flour mixed with vinegar! Some reactions are spontaneous, meaning they proceed readily when the reactants are mixed together, and some are not. The Gibbs Free Energy of reaction (DG) is an indicator of reaction spontaneity. In other words, if we know the DG of a reaction, we know whether the reaction is thermodynamically favorable. For example, in section 2 of this tutorial, we examined a diagram of reaction with a negative DG:
Recall that a reaction with a negative DG (where the products are at a lower energy than the reactants) is thermodynamically favorable. Obeying the second law of thermodynamics, naturally occurring reactions always move toward a state of lower potential energy. Thus, a reaction with a negative DG, like the one in the diagram, is said to be spontaneous. In the same way, a reaction with a positive DG (a reaction where the energy of the products is higher than the energy of the reactants), will not spontaneously occur. Remember, just because a reaction has a positive DG does not mean that the reaction is completely impossible. It just means that it will not occur without the appropriate energy input. For example, many physiological reactions that build biomolecules have a positive DG but occur with great frequency in our bodies nonetheless, because they are linked to, and thus fueled by, other reactions that make free energy available. So what happens when DG is zero? In that case, the DG is not negative, so the reaction doesn’t spontaneously occur. However, the DG is also not positive, so the reaction also doesn’t need energy input to proceed. As you might imagine, such a reaction just sits there, without undergoing any net change in the levels of reactants or products. (We will return to this point in a later section.) A reaction that has already proceeded as far as it will go is said to be at equilibrium. This table summarizes the correlation between DG and reaction spontaneity.
|
||||||||||||||||||||||||