|
THE EFFECTS OF TEMPERATURE AND CONCENTRATION ON REACTION RATESWe have seen that reaction rates are determined experimentally for different chemical reactions. But what influences whether a reaction proceeds quickly or slowly? To answer that question, we need to think about the individual molecules involved in the reaction. The rate of a chemical reaction is determined by how the molecules involved in the reaction move. In other words, the kinetics of the reaction are influenced by how quickly the molecules are moving, and how often and in what orientation the molecules bump into one another. Collision theoryThe study of kinetics involves knowing something about collision theory, which involves thinking about how molecules crash into one another. Collision theory tells us that molecules have to click together just right, with enough speed to latch together. If they are moving too slowly, there is not enough energy available to break existing bonds, and if they are not facing each other the right way, the new bonds won’t be able to form. It’s like being at a supermarket parking lot. The faster people looking for a parking spot are driving, the more likely they will crash into each other. We already know from our look at rate equations that chemical reactions are affected by the concentration of the reactants. Collision theory is helpful in understanding why this is so. Since the molecules have to physically contact one another for a chemical reaction to occur, it makes sense that the more molecules are present, the more likely they are to bump into one another. Back to our supermarket parking lot analogy: You are much more likely to bump into another car if there are lots of cars circling the lot looking for a spot than if the parking lot is nearly empty. Temperature effects
Understanding collision theory also makes it easier to understand why temperature
affects the kinetics of chemical reactions. In a population of molecules, some
have more energy than others. Just like cars on a busy freeway, some molecules
are moving very fast, some are very slow, and most are going around the same average
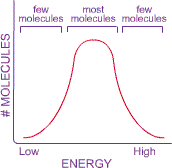
speed. This idea is represented in a graph that shows the number of molecules
at each energy level.
Moving slowly is a good thing in that crowded supermarket parking lot, since
a collision at low speed won’t even dent the license plate. Similarly, two
slowly moving molecules will just bounce off each other without reacting. But
with a bit more speed there is more energy that can result in things breaking
when they crash. At higher temperatures, molecules move faster. Thus, it makes
sense that more of the molecules will have enough energy to break some bonds when
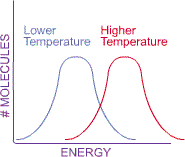
they collide. An increase in the temperature thus increases the average energy
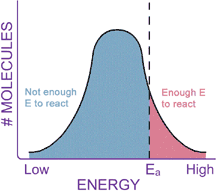
of the molecules, shifting the energy curve to the right. This shift in the energy distribution curve is important because it also helps explain why reactions occur faster when the temperature rises. Remember that the activation energy is the energy wall that has to be scaled by the reactant molecules in order to form the products. Each chemical reaction has a certain activation energy, and only molecules with at least this much energy can react. At a lower temperature, a smaller proportion of the molecules will have enough energy to scale the wall (portion of the diagram below shaded in red). The molecules in the blue shaded area of the curve don’t have enough energy to react. Raising the temperature shifts the curve to the right, so that a larger proportion of the molecules now have enough energy to react.
Now that we’ve seen how the speed of the molecules affects reaction rates, let’s examine how the orientation of the molecules influences a chemical reaction. Let’s look at the molecules involved in the following reaction:
If the molecules are not facing each other properly, they will not react. However, if they are facing each other in the correct orientation, and are moving with enough speed, the reaction will occur.
|
|||||||||||||||||||||||||||||||