|
KINETICS IN BIOCHEMISTRY: THE ACTION OF CATALYSTSVirtually all of the chemical reactions that occur in our bodies are catalyzed by enzymes. Without enzymes, these reactions would occur too slowly to be biologically relevant. It is important to understand what an enzyme can do, and what it cannot do. What enzymes can do, and do very well, is speed up reactions. For instance, consider the hydrolysis of peptide bonds, which link amino acids together into proteins. Enzymes are biological catalystsThe rate of peptide bond hydrolysis in water has been calculated at 10–9 s–1. This is equivalent to about one bond breaking every 20 years. However, enzymes can increase this rate up to about 190 s–1 (that’s a 100 billion-fold increase!). In general, enzymes increase reaction rates by many orders of magnitude.
How do enzymes accomplish this feat? Consider a well-studied reaction, the decomposition of hydrogen peroxide into water and oxygen:
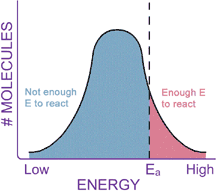
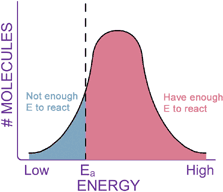
This reaction will occur spontaneously without a catalyst, but it happens much faster in the presence of the enzyme catalase. Notice how the activation energy (DG‡, or Ea) of the catalyzed reaction is much lower than that of the uncatalyzed reaction. The enzyme physically helps the molecule through the transition state, like a big hand molding silly putty. Thus, an enzyme increases the speed of a chemical reaction by lowering the activation energy of the reaction. When the Ea is lowered, a larger portion of molecules in the reaction have enough energy to overcome this barrier and react.
Enzymes are not so magical that they can change all the properties of a chemical reaction. In fact, about all they can do is speed up the reaction rate. Enzymes cannot change whether a reaction is thermodynamically favorable. If a reaction does not proceed because it is not thermodynamically favorable (that is, it has a positive DG), adding an enzyme will not make the reaction "go." Similarly, the Keq of a reaction does not change when an enzyme is added. Remember that the Keq tells us how much product will be present when the reaction is at equilibrium. Recall the "fruits to nuts" reaction from the previous section, which had a Keq of 2.
|
||||||||||||||||||||||||||

 At
equilibrium there will be twice as many fruits as nuts (here there are six nuts
and three fruits). If you discovered a new enzyme, "fruitase" to catalyze
the reaction, you would be able to reach equilibrium much faster. However, when
the "fruitase"-catalyzed achieves equilibrium, you will still have six
nuts and three fruits.
At
equilibrium there will be twice as many fruits as nuts (here there are six nuts
and three fruits). If you discovered a new enzyme, "fruitase" to catalyze
the reaction, you would be able to reach equilibrium much faster. However, when
the "fruitase"-catalyzed achieves equilibrium, you will still have six
nuts and three fruits.