REDOX REACTIONS
What is a redox reaction?
As indicated by its name, the redox (oxidation-reduction) reaction is composed of two parts: an oxidation half reaction and a reduction half reaction.
 These two seemingly opposed reactions are both needed–there can be no oxidation without a concomitant reduction and vice versa. These half reactions are the Yin and Yang of redox chemistry. These two seemingly opposed reactions are both needed–there can be no oxidation without a concomitant reduction and vice versa. These half reactions are the Yin and Yang of redox chemistry.
To better understand what each of these half reactions entails, let’s
use a common redox reaction as an example. It would be hard to imagine life today
without the combustion of hydrocarbon fuels. Natural gas, or methane, is a common
fuel used to power hot water heaters, warm homes, and run gas stoves.
| CH4 |
+ |
2O2 |
 |
CO2 |
+ |
2H2O |
+ |
HEAT |
| methane |
|
oxygen |
|
carbon
dioxide |
|
water |
|
energy |
|
|
Redox reactions frequently involve changes in bonds to oxygen
The term “oxidation” suggests that oxygen is somehow involved in
the oxidation half reaction. The carbon atom in methane loses hydrogen atoms
but gains oxygen atoms. By gaining oxygen atoms, the carbon is oxidized. Meanwhile,
the oxygen molecule (O2) loses an oxygen atom but gains hydrogen
atoms. By losing oxygen atoms, the O2 molecule is reduced.
Thus, one common definition of oxidation is “the gaining of oxygen” while the concomitant reduction reaction can be defined as the “losing of oxygen.” More specifically, oxidation can be considered “an increase in the number of bonds to oxygen” and reduction “a decrease in the number of bonds to oxygen.” However, while these definitions serve to identify the oxidized and reduced components of many organic reactions, they cannot be applied to all redox reactions.
| Oxidation: an increase in the number of bonds
to oxygen |
| Reduction: a decrease in the number of bonds to
oxygen |
|
Redox reactions are electron transfer reactions
Each atom involved in a redox reaction can be assigned an “oxidation state”
to help keep track of the movement of electrons for the reaction. Remember that
all atoms consist of a nucleus (containing neutrons and positively charged proton
particles) with orbiting negatively charged electron particles (the exception
is hydrogen, which lacks neutrons and consists only of a proton and an electron).
When atoms react, electrons are often transferred from one atom to another or
are shared by more than one atom. Changes in oxidation states tell us whether
atoms that have reacted have donated or accepted electrons.
| Redox reactions involve the transfer of electrons |
|
Thus, a more general definition of redox reactions involves the transfer of
electrons. The compound that donates electrons is being oxidized, and the compound
that accepts the electrons is being reduced.
 |
Cu(s) + 2Ag+(aq)
 Cu2+(aq) + 2Ag(s)
Cu2+(aq) + 2Ag(s)
|
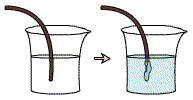
With this definition, even reactions that do not involve oxygen can be redox reactions. For example, when copper wire is dipped into a solution of silver nitrate (AgNO3), the clear solution becomes blue over time, and the copper wire becomes coated with silvery needles (the silver nitrate solution consists of Ag+ and NO3– ions). This result is due to the transfer of electrons between copper and silver.
The copper atoms donate electrons to the silver ions in solution. As a result,
the copper ions become positively charged and go into solution. The silver cations
in solution accept the electrons, and become uncharged solid silver atoms that
deposit onto the copper wire.
The most useful definition of a redox reaction is the most general one which
simply involves the transfer of electrons. In addition, memorizing the simple
mnemonic “OILRIG” can help you identify
the oxidized and reduced components of a redox reaction.
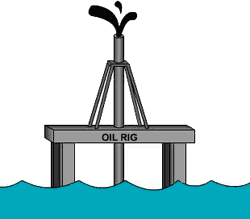 |
OIL RIG
Oxidation
Is
Loss (of electrons)
Reduction
Is
Gain (of electrons)
|
You can use this mnemonic to determine the oxidized and reduced components
of a redox reaction:
Cu(s) + Ag+  Cu2+ + Ag(s)
Cu2+ + Ag(s) |
|
The solid copper atoms (Cu0) lost negatively charged electrons, thus becoming positively charged Cu2+ ions. Since the copper atoms lost electrons, the copper is oxidized. At the same time, the positively charged silver ions each gained a negatively charged electron and became insoluble, solid silver. Since the silver atoms gained electrons, the silver is reduced.
In the above reaction, identifying which atoms gained and lost electrons is
straightforward. However, tracking the electrons can be a bit more difficult in
reactions where the ions are not identified. There are several simple rules for
figuring oxidation states of compounds that will allow you to identify the oxidized
and reduced substances in a reaction:
- All uncharged elements and compounds have an oxidation state of zero. Examples are Zn, H2, O2, and KMnO4.
- All charged elements and compounds have an oxidation state equal to their charge.
- Oxygen in a compound almost always has an oxidation state of –2.
- Hydrogen in a compound almost always has an oxidation state of +1.
- Some elements always have the same oxidation states when they are in a compound. These include “group 1” elements such as H, Li, Na, K (always +1), “group 2” elements such as Mg and Ca (always +2), and “group 7” elements such as F and Cl (always –1).
Example 1

In the redox reaction
2H2 + O2  2H2O 2H2O
which atoms have become oxidized, and which are reduced?
Answer:
From rule 1, you know H2 has an oxidation state of 0, therefore each H atom in H2 has an oxidation state of zero.
From rule 1, you know O2 has an oxidation state of 0, therefore each O atom in O2 has an oxidation state of zero.
From rule 3, you know any H in a compound (here H2O) has an oxidation state of +1.
From rule 4, you know any O in a compound (here H2O) has an oxidation state of –2.
Thus, you can write the oxidation state over each atom in the reaction as below:
| (0) |
|
(0) |
|
(+1)(–2) |
| H2 |
+ |
O2 |
 |
H2O |
The hydrogen atoms have gone from an oxidation state of zero to +1 and have thus lost negatively charged electrons. The hydrogen atoms have been oxidized. At the same time, the oxygen atoms have gone from an oxidation state of zero to –2, and have thus gained electrons (the oxygen atoms have been reduced). Notice that the overall oxidation state of the compound H2O, containing two hydrogens (two times +1) and one oxygen (–2) is zero, satisfying rule 1.
|