|
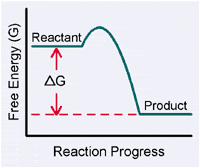
GIBBS FREE ENERGY OF REDOX REACTIONSIn the preceeding section, we learned that DE°' is the change in the reduction potential of a redox reaction. We also were shown that a positive DE°' value indicates a spontaneous reaction. Changes in reduction potential are linked to changes in the free energy of a systemThe field of thermodynamics is concerned with the flow of energy during chemical reactions. You should be familiar with the basic concepts of thermodynamics from general chemistry, but if you need to refresh your knowledge please visit the Thermodynamics Review. One of the core concepts of thermodynamics is that for any spontaneous reaction, free energy is lost. Spontaneous reactions always proceed “downhill” to create products containing less free energy than the reactants. This idea is expressed with a quantity called the Gibbs Free Energy change or DG°'.
The Gibbs Free Energy change (DG°') is the quantity of usable energy (chemical potential) in a reaction. Here, DG°' is the difference between the free energy of the reaction products and the reactants:
Since naturally occurring or “spontaneous” processes always proceed toward the state that has the least potential energy, the energy of the products is less than the energy of the reactants.
It is not surprising then that changes in the quantities of Gibbs Free Energy and reduction potential, DE°' and DG°', are related to one another, for both can be used to predict whether processes are spontaneous. Indeed, the relationship between these two quantities is given by the following formula:
Notice that the relationship between DG°' and DE°' is an inverse one: when DE°' is positive, DG°' is negative, and vice versa. This makes sense because for spontaneous redox processes DG°' is negative and DE°' is negative. Example 5
|
|||||||||||||||||||||||||||||||
| malate + NAD+ |
The two half reactions from the half reaction table are:
| (1) | oxaloacetate + 2 H+ + 2 e– |
E°' = –0.175 V |
| (2) | NAD+ + 2H+ + 2 e– |
E°' = –0.320 V |
First, we need to find the DE°' of the reaction:
The overall reaction consists of half reaction (2) added to the reverse of half reaction (1):
| malate + NAD+ + 2H+
+ 2e– |
Looking at the overall reaction, we can see that half reaction (2) functions as the reduction reaction (NAD+ gains electrons), and half reaction (1) functions as the oxidation reaction (malate loses electrons). The DE°' for the reaction is therefore
| DE°'
= (E°' from reduction reaction) –
(E°' from oxidation reaction) |
Then, use the formula to calculate DG°' :
| DG°' = –nF(DE°') |