|
STRONG AND WEAK ACIDSIonizationThere are two classes of acids: strong acids and weak acids. Note that strong acids and bases are rarely a concern in biochemistry (as they are in general chemistry). Strong acids ionize (break into ions) completely when dissolved in water. In other words, a strong acid is a good proton donor. For example, the strong acid HCl gives up all its protons to water:
There are only a few strong acids, which are all listed below. HCl (hydrochloric acid, found in stomach digestive juices) is a good example of a strong acid.
Because strong acids completely ionize, the [H+] of a solution made with a strong acid is easily figured out, since it is equal to the molarity of the solution. Thus, a 0.1 M solution of HCl has a [H+] of 0.1. A 0.029 M solution of HCl has a [H+] of 0.029 and so on. (Remember that this only holds true as long as the molarity of your solution is somewhat greater than 10–7 M. Otherwise, for solutions so dilute that the H+ normally present in pure water is comparable or larger, you will need to take the [H+] of pure water into consideration.) Similar to the case with strong acids, there are just a few strong bases that ionize completely. For example, when sodium hydroxide ionizes in water, the resulting hydroxide ion readily accepts a proton:
The strong bases are listed in the table below.
Most acids of biological origin are weak. In part 1 of this tutorial, we saw that in pure water, a very small percentage of the H2O molecules dissociate to form H+ and OH– ions. Most of the molecules in pure water remain intact as H2O. The same can be said of weak acids. When weak acids are dissolved in water, only some of the acid molecules actually dissociate (break apart), while most others remain intact. 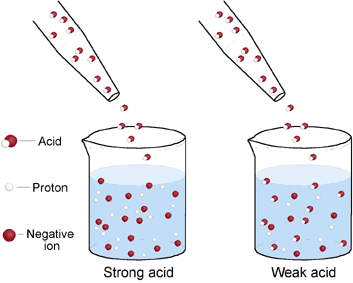
The percentage of the molecules that break apart depends on the weak acid. Some weak acids dissociate very minimally, and as a consequence such an acid has little acid strength. Other weak acids dissociate more, and as a consequence have more acid strength. The degree of dissociation of a weak acid in water is described by the acid dissociation constant, Ka. For the dissociation of acetic acid (the acid in vinegar) in water,
the equilibrium (or acid dissociation) constant expression for the reaction is given by
(Remember that by convention, [H2O] is left out of the expression because the reaction is taking place in water). The “a” subscript on Ka is a reminder that this number represents an acid dissociation constant. The larger the Ka, the more the acid dissociates (or reacts with water) to form H3O+ ions, and the stronger the acid. Note how Ka values for weak acids tend to be very small numbers. Because these numbers with exponents tend to be cumbersome, Ka values are usually converted to pKa values, much in the same way as [H+] values are converted to pH.
For example, the Ka of acetic acid is 1.74 x 10–5. The pKa for acetic acid is therefore pKa = –log (1.74 x 10–5) = 4.76. Below is a table listing some common weak acids and their Ka and pKa values.
Note that the smaller the Ka, the larger the pKa. Thus, stronger acids are represented by larger Ka values, but smaller pKa values. Also, whether an acid is strong of weak can be readily identified by either its Ka or pKa:
Hydronium acid (H3O+), which is the protonated form of water, is the dividing line between a strong or weak acid. In other words, a strong acid is defined as one that ionizes to a larger degree than the H3O+ acid form of water does (Ka = 1, pKa = 0). Another way to put this is that if you put a strong acid in water, most of it ionizes, while for a weak acid most stays in its conjugate acid (protonated) form. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||