| |
ACIDS AND THEIR CONJUGATE BASES
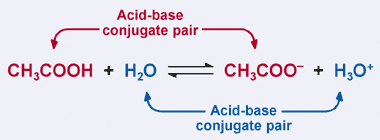
Remember the Bronsted–Lowry definition of an acid: an acid is a proton
donor. Clearly, in our previous example acetic acid donates its proton to H2O
in the forward reaction, resulting in the formation of the acetate ion. However,
in the reverse reaction, the acetate ion accepts a proton from H3O+
to revert to CH3COOH. From the Bronsted–Lowry definition, a base
is a proton acceptor.
| CH3COOH |
+ |
H2O |
  |
CH3COO– |
+ |
H3O+ |
| acetic
acid |
|
water |
|
acetate
ion |
|
hydronium
ion |
|
Thus, the acetate ion acts as a base in the reverse reaction, and is called
the conjugate base of acetic acid. Acetic acid and the acetate ion are known as
an acid–base conjugate pair. Interestingly enough, there is a second acid–base
conjugate pair in the reaction above. Notice how in the reverse reaction, H3O+
donates a proton (making it an acid), while in the forward reaction, H2O
accepts a proton (making it a base). H2O and H3O+
are a conjugate acid–base pair, with H3O+ being the
conjugate acid and H2O being the conjugate base.
 |
| In a conjugate acid–base pair, the conjugate acid
has one more proton than its corresponding conjugate base. |
|
